- Purpose
- Typical Anticancer
Rituxan
When a Nobel Prize-winning technique for using mouse cells to mass produce highly specific monoclonal antibodies (mAbs) was first developed in the 1970s, there was great hope for mAbs as magic bullets that would target cell receptors associated with a range of diseases. These hopes were largely dashed when early mouse-based mAbs proved either ineffective or harmful.
 COURTESY OF ANTONIO GRILLO-LÓPEZ COURTESY OF ANTONIO GRILLO-LÓPEZ |
|
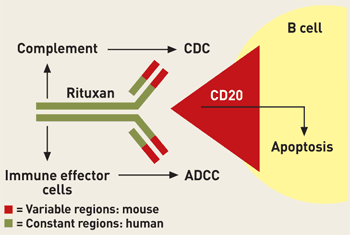
KILLER Rituxan kills B cells associated with non-Hodgkin's lymphoma by binding to the cells' CD20 receptor and thus inducing one or more of three mechanisms--complement-dependent cytotoxicity (CDC), antibody-dependent cell-mediated cytotoxicity (ADCC), and/or apoptosis. |
The situation for mAbs has since turned around almost completely with about 20 now approved in the U.S. and approximately 100 currently in testing for a diverse set of medical conditions. A turning point for mAbs was the successful development of Rituxan, the first therapeutic antibody approved for cancer.
"Rituxan's story is truly great," says hematologist and oncologist Antonio J. Grillo-López, who managed clinical development and regulatory affairs leading to approval of the drug. Rituxan was one of the first drugs to fulfill early predictions about the therapeutic potential of mAbs, and its development sparked renewed interest in mAb R&D, he says.
Non-Hodgkin's lymphoma, the disease Rituxan is approved to treat a family of cancers of lymphoid tissue, including lymph nodes, bone marrow, and spleen. It's the 10th most prevalent cancer worldwide. Nevertheless, Rituxan is currently the largest selling cancer drug in the U.S., in part because of its off-label use for treatment of other conditions for which it has been found to be effective, including leukemia and autoimmune conditions such as lupus, rheumatoid arthritis, and myasthenia gravis. Rituxan had U.S. sales of about $1.6 billion in 2004.
DEVELOPMENT OF Rituxan can be traced back to 1979, when hematologist and oncologist Lee M. Nadler of Dana-Farber Cancer Institute, Boston, developed a mAb that bound to CD20, a cancer-associated receptor on B cells (antibody-producing immune cells). Researcher Nabil Hanna and coworkers at IDEC Pharmaceuticals (now Biogen Idec), Cambridge, Mass., developed a related mAb in the laboratory, and clinical trials of the resulting drug, Rituxan, were then managed by Grillo-López.
"I discovered the antibody and antigen, and Antonio and IDEC made it a drug," Nadler says. "I am very happy that Rituxan has helped so many patients."
Nadler was also the first person to administer a mAb to lymphoma patients--using a mouse-based mAb that was later radiolabeled with iodine and became GlaxoSmithKline's Bexxar. But Rituxan received approval earlier--in 1997, versus 2003 for Bexxar--thus becoming the first anticancer mAb. Rituxan is now marketed by four companies--Genentech, Biogen Idec, Hoffmann-La Roche, and Zenyaku Kogyo. Genentech provided major financial support for development of the drug, and "as a result, today it owns 70% of Rituxan," Grillo-López says.
The mAb-generating technique described in 1975 by Georges J. F. Köhler and César Milstein at the Laboratory of Molecular Biology in Cambridge, England--for which they shared the 1984 Nobel Prize in Physiology or Medicine--was carried out in mouse cells. Therefore, early mAbs were entirely mouse-based. Some mouse-based mAbs proved to be problematic in clinical trials because they were rejected by the human immune system, causing adverse reactions and even death.
Researchers tried to address the problem by making mAbs more human and less mouselike. Initially, they developed chimeric mAbs, in which only the antibody's variable domains (the parts that vary in sequence from one antibody to another and determine its specificity) were mouse-based, whereas the rest of the antibody (its constant regions) were human. In an effort to make mAbs even less immunogenic, scientists subsequently developed humanized mAbs, in which the amount of mouse-based sequence was restricted to the complementarity-determining regions (small segments of the variable domains). And they later developed fully human mAbs, which contain no mouse sequence whatsoever.
Rituxan is chimeric. The IDEC group genetically engineered human constant regions into the original mouse-based version of the antibody to decrease its immunogenicity and enable it to recruit immune-system cytotoxic mechanisms. Some patients do generate antibodies against Rituxan, but this doesn't seem to caused significant side effects.
The drug's mode of action is to bind to the CD20 antigen on surfaces of normal and malignant B cells. It then destroys B cells by inducing one or more of three immune cell-killing mechanisms. Stem cells (B-cell progenitors) in bone marrow lack the CD20 antigen, allowing healthy B cells to regenerate after treatment and return to normal levels within several months.
Rituxan has had a significant impact on outcomes for lymphoma patients.
Nevertheless, the search for even better mAb lymphoma drugs continues. "This is one of the more promising technologies in the area of cancer therapeutics," Grillo-López says.—

