Science & Technology
March 31, 2008 - Volume 86, Number 13
- pp. 26-27
Science & Technology Concentrates
- A Squishy Squid And Its Tough Beak: Mystery Resolved
- Minimizing Halogenated Mutagens In Water
- Novel Fenestranes Stitched Together
- Humans Make Up For Vitamin C Shortfall
- Control System With An Enzyme PIN
- Oceanographers Gain A Tracer Compound
- Graphene's Electronic Limits Revealed
- Alternative Route To Dehydroalanine
A Squishy Squid And Its Tough Beak: Mystery Resolved
 Science © 2008
Science © 2008 How the Humboldt squid avoids harming its own soft, gelatinous body when it wields its tough beak to attack and eat prey is now known. The tip of the razor-sharp beak is among the world's hardest and stiffest purely organic biomaterials. But the beak attaches to the squid's muscular cheek tissue, which "has the consistency of Jell-O," says Francis W. Zok of the University of California, Santa Barbara. "You can imagine the problems you'd encounter if you attached a knife blade to a block of Jell-O and tried to use that blade for cutting. The blade would cut through the Jell-O at least as much as the targeted object." Zok, J. Herbert Waite, and colleagues discovered that the beak's stiffness gradually diminishes between the tip and the base. The base is 100 times more flexible than the tip, which reduces the impact on the cheek tissue near the beak's base (Science 2008, 319, 1816). The researchers found that the degree of stiffness at different points correlates with the ratio of chitin, water, and proteins cross-linked with 3,4-dihydroxyphenyl-L-alanine.
Minimizing Halogenated Mutagens In Water

Halogenated furanones can be produced in drinking water by the reaction of naturally occurring organic matter and bromide with the disinfectant chlorine. Although halogenated furanone compounds are mutagenic, research on their concentrations in drinking water is at an early stage and they are not regulated by EPA. Gretchen D. Onstad of the University of Washington, Seattle; Howard S. Weinberg of the University of North Carolina, Chapel Hill; and Stuart W. Krasner of the Metropolitan Water District of Southern California now report how the formation of halogenated furanones can be affected by different water-treatment methods (Environ. Sci. Technol., DOI: 10.1021/es071374w). The group studied water from six pairs of treatment plants in which each member of a pair used a different scheme to treat water. The researchers used gas chromatography with microelectron capture detection to analyze the samples for 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone (shown), known as MX, as well as a dozen MX analogs. The highest levels of halogenated furanones, as much as 1 μg/L of one of the analogs, were produced when disinfection began with chlorine or chloramine. Treatment plants produced much less of the targeted compounds when they used ozonation, which is thought to degrade furanone precursors, followed by filtration and then chlorination or chloramination.
Novel Fenestranes Stitched Together

Using a one-pot procedure, chemists in France have developed a simple synthesis of complex [4.6.4.6]fenestradienes and [4.6.4.6]fenestrenes (J. Am. Chem. Soc., DOI: 10.1021/ja800691c). The efficient method provides rapid access to these unusual molecular scaffolds, which could be used as materials, ligands for catalysis, and pharmaceuticals. Named after the Latin word for window, fenestranes feature a windowpane-like structure in which four rings share a quaternary carbon. Building these polycycles' strained structures poses a challenge for synthetic chemists. Using a trienyne as their starting point, Jean Suffert, Catherine Hulot, and Gaëlle Blond of Louis Pasteur University of Strasbourg were able to design a cyclization cascade that assembles a [4.6.4.6]fenestrane skeleton with good yields (shown). In the key step, a nickel catalyst partially reduces the trienyne's triple bond. The researchers believe that the tetraene generated in this reaction undergoes an 8π-conrotatory electrocyclization followed by a 6π-disrotatory electrocyclization to form a [4.6.4.6]fenestradiene. This highly reactive compound (center) readily oxidizes in air to form a [4.6.4.6]fenestrene (right). "The high yields and the limited number of steps of the reaction sequence render this process very attractive for the synthesis of new fenestranes," the authors write.
Humans Make Up For Vitamin C Shortfall

More than 4,000 mammals can make vitamin C from glucose, but the human version of the necessary enzyme is out of order. To make up for our species' incompetence, humans have evolved a way to efficiently utilize the vitamin C we consume. In particular, researchers led by Naomi Taylor at the National Center for Scientific Research, in Montpellier, France, show that a glucose transport protein on the surface of human red blood cells is altered by another protein called stomatin so that vitamin C's oxidized form, L-dehydroascorbic acid (DHA, shown above right), is preferentially imported into red blood cells instead of glucose (Cell 2008, 132, 1039). DHA is then quickly reduced to vitamin C inside the blood cells. In fact, humans express three orders of magnitude more of these glucose-turned-DHA transporters on their red blood cells than do mammals that produce their own vitamin C. Because of this efficient vitamin C cycling, humans need only consume 1 mg of vitamin C per kg of body weight per day, whereas goats have to synthesize 200 times that amount.

Control System With An Enzyme PIN
A security system with a personal identification number (PIN) made of enzymes rather than numbers or letters has been devised by Evgeny Katz and coworkers at Clarkson University, Potsdam, N.Y. This type of biochemical network, which mimics an electronic keypad lock, could someday be used to control medical devices based on individual body chemistry (J. Am. Chem. Soc., DOI: 10.1021/ja7114713). Another group has made this type of device before by using a synthetic pathway, but because the new system is made from biochemical components "it offers advantages in terms of biocompatibility," says molecular electronics expert Devens Gust of Arizona State University. Katz's prototype system is a sucrose solution containing a dye. The dye can be oxidized to a measurable colored output, but only after a sequence of three enzymes is added to the solution in the correct order (shown). It's too early to know whether this system might be used for releasing drugs in response to a specific sequence of biochemical events, Katz says. For now, the team is working on reconfiguring enzyme networks to process biochemical information in different ways.
Oceanographers Gain A Tracer Compound
Oceanographers have traditionally injected kilogram amounts of sulfur hexafluoride gas into the ocean and followed the evolution of these spikes by gas chromatography of water samples to study mixing processes. The technique is useful because SF6 can be detected at femtomolar quantities and tracked over large areas and long time periods. But researchers need a different tracer for localized studies because SF6 must be reserved for global studies of ocean circulation and uptake of anthropogenic CO2. Columbia University's David T. Ho and colleagues now report that the SF6 analog SF5CF3 fits the bill (Geophys. Res. Lett., DOI: 10.1029/2007GL032799). The team conducted a two-year experiment in the Santa Monica Basin off the coast of Southern California that shows SF5CF3, which is not commercially available yet, can be detected almost as well as SF6 and doesn't interfere with using SF6 for simultaneous tracer studies. Both compounds are greenhouse gases, but the researchers write that the amounts of tracer gases used in ocean studies are insignificant and that SF5CF3 is an improvement because its atmospheric lifetime is one-fourth that of SF6.
Graphene's Electronic Limits Revealed
Physicists at the University of Maryland have thoroughly probed the conductivity of graphene—a single-atom-thick sheet of graphite—and measured key parameters that for the first time give a complete picture of the current limitations and future promise of the recently discovered electronic material (Nat. Nanotechnol., DOI: 10.1038/nnano.2008.58). Jian-Hao Chen, Michael S. Fuhrer, and coworkers report values for the resistivity (resistance to electron flow) and mobility (how fast electrons move) of graphene samples on a silicon dioxide substrate. But because graphene is just a single layer of carbon atoms, the researchers found that its very low resistivity and very high mobility are impacted by electronic interactions not only within the graphene layer but also with the substrate beneath. They anticipate that with better sample preparation techniques and less interactive substrates—silicon carbide and diamond are promising candidates—graphene's resistivity and mobility can be improved to near the material's room-temperature limits. The researchers showed that those values can surpass the low resistivity of silver and the high mobility of InSb, the current record holders, and put graphene's mobility about 100 times higher than that for silicon.
Alternative Route To Dehydroalanine
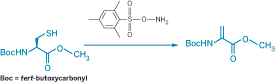
Dehydroalanine (Dha), which can be formed by oxidative elimination of alkylated derivatives of cysteine (Cys) or selenocysteine, provides a chemical handle for selectively modifying proteins and thereby studying the process of posttranslational modifications. The usual strategies for forming Dha rely on peroxide-induced oxidative elimination, which can unintentionally affect methionine as well. Benjamin G. Davis and coworkers at the University of Oxford instead use O-mesitylenesulfonylhydroxylamine (MSH) for the oxidative elimination reaction (J. Am. Chem. Soc., DOI: 10.1021/ja800800p). When the researchers treated Boc-protected Cys methyl ester with excess MSH (shown), the Dha derivative formed at almost quantitative yield in just a few minutes at room temperature in open air. They used the strategy to convert the single exposed Cys on the surface of subtilisin from the bacterium Bacillus lentus without oxidizing any of the three methionine units also present. The researchers then tethered various posttranslational modifications to the protein by adding thiols to the Dha. In one case, they regenerated Dha from the resulting thioether by a similar oxidative elimination using MSH, allowing a "functional switch" on the protein surface.
- Chemical & Engineering News
- ISSN 0009-2347
- Copyright © 2011 American Chemical Society